Real-world Data Demonstrates Successful Transitions from Humira to Biosimilars
Authors: Justin Arzt, PharmD; Agata Siwak, PharmD, MSBA; Marnie Wickizer, PharmD, AE-C, CDCES; Ryan Schmidt, PharmD; Robert Topp, RN, PhD; Matt Hustad, PharmD
All authors are employees of Navitus Health Solutions.
Abstract
Adalimumab biosimilar adoption through formulary preference offers a straightforward strategy for lowering pharmaceutical expenditure. However, the impact on utilization, treatment discontinuation or patient experience that results from transitioning patients from reference product Humira® (adalimumab) to a biosimilar is poorly understood. To investigate this further, pharmacy claims data was compared for members of three commercial health plans using a specialty pharmacy. Results suggest formulary changes are effective in promoting utilization with minimal discontinuation. Additionally, there were less reported adverse events as well as significant plan and copay savings. This paper was first presented at the AMCP 2025 conference in Houston, Texas, on April 2, 2025.
Featured Quote
The dramatic 97% reduction in average copays observed in the Navitus adalimumab transition program demonstrates the effectiveness of the specific formulary designs and benefit structures implemented. These savings were achieved while maintaining high treatment continuation rates and patient satisfaction, demonstrating that cost reduction need not come at the expense of clinical outcomes (Arzt, Siwak, Wickizer, et al, 2025, p. 10).
Executive Summary
The introduction of adalimumab biosimilars represents a significant opportunity to reduce healthcare costs while maintaining therapeutic efficacy. This study examines real-world clinical outcomes from a structured program conducted by Navitus to transition patients from the reference product Humira® (adalimumab) to FDA-approved biosimilar alternatives. Based on comprehensive research conducted across multiple commercial health plans, the findings demonstrate high rates of successful transition, substantial cost savings and minimal clinical impact — providing valuable insights for healthcare decision-makers considering biosimilar adoption strategies.
Introduction
Humira (adalimumab) has been one of the most profitable drugs in pharmaceutical history, with global cumulative sales exceeding $219 billion since the ground-breaking anti-inflammatory medication therapy launched. As the most widely prescribed tumor necrosis factor (TNF) inhibitor for autoimmune conditions, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease and ulcerative colitis, Humira has revolutionized treatment outcomes – but at a significant cost to healthcare systems.
The January 2023 introduction of adalimumab biosimilars in the U.S. market created new possibilities for cost containment while maintaining quality care. A biosimilar is a biological product highly similar to an FDA-approved reference (brand) product, with no clinically meaningful differences in terms of safety, purity and potency. Unlike generic small-molecule drugs, biosimilars are complex proteins manufactured in living systems, requiring rigorous comparative assessment to ensure therapeutic equivalence.
Despite the availability of biosimilars, uptake has been slower than anticipated in the United States compared to European markets. This lag is largely attributable to complex rebating strategies, hesitancy among providers and patients to switch from established treatments and misaligned incentives between manufacturers, payers and pharmacy benefit managers (PBMs).
To address these barriers and generate real-world evidence to inform broader adoption, a comprehensive study was conducted to evaluate clinical outcomes, patient experience and economic impact of a structured adalimumab biosimilar transition program.
Study Design and Methodology
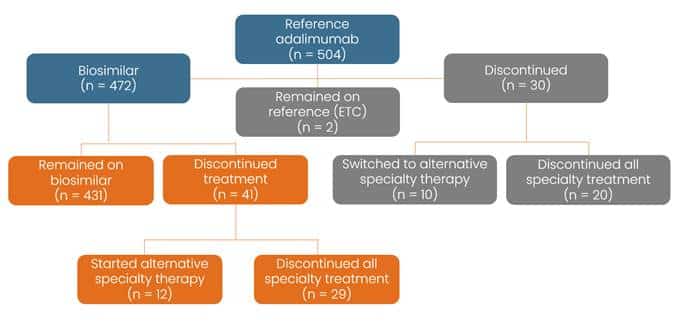
The research examined outcomes for patients across three commercial health plans transitioning from Humira to adalimumab biosimilars, as part of the Navitus PBM adalimumab biosimilar transition program. Prescription fulfillment was managed through either Lumicera Health Services or another specialty pharmacy. The study included 504 eligible patients who had:
- At least six months of adalimumab fill history (establishment period) prior to the June 1, 2024, formulary change
- Fill history of both Humira and at least one adalimumab biosimilar product
- Less than three months of fill gaps in claims history prior to transition
- Active benefit coverage throughout the study period
This six-month establishment period considered findings from previous literature that patients, when first starting adalimumab therapy, have an attrition rate of 40-46% within the first few months. By selecting patients already established on therapy for at least six months, researchers minimized the confounding variable of natural adalimumab discontinuation that occurs early in treatment.
Investigators analyzed multiple outcome domains:
- Utilization (Primary objective): Rates of biosimilar adoption and specific product selection
- Attrition (Secondary objective): Reasons for discontinuation and rates of switching to alternative treatments
- Member Impact (Secondary objective): Patient satisfaction, out-of-pocket costs, therapy gaps, adverse events and specialty pharmacy interventions
This comprehensive approach allowed researchers to assess both the quantitative success rates of the transition program and the qualitative factors affecting patient experience and clinical outcomes.
Key Findings
Biosimilar Adoption and Utilization
The study demonstrated remarkably high rates of successful transition from Humira to biosimilar alternatives:
- 94% of members successfully switched to a biosimilar within three months of the formulary change
- 77% of patients initially transitioned to adalimumab-adaz (Hyrimoz equivalent)
- 8% first used adalimumab-fkjp (Hulio equivalent)
- 6% of patients switched to a different adalimumab biosimilar after their initial transition
- 3% of patients used adalimumab-ryvk (Simlandi), one of the few interchangeable biosimilars not requiring a new prescription
- Only 0.4% of patients (two members) received exceptions to coverage to continue Humira treatment
Overall, the high transition rate (94%) indicates strong acceptance of the formulary change implementation approach, which included proactive patient communication, provider education and care coordination across the healthcare team. Transition differences observed between client groups indicate some impact of variable provider engagement and communication channels. Collectively, these findings underscore the importance of both clinical evidence and practical implementation strategies in successful biosimilar adoption.
The prevalence of adalimumab-adaz (Hyrimoz) as the most commonly used biosimilar may be attributable to several factors. Hyrimoz was studied in both psoriasis and rheumatoid arthritis clinical trials with crossover designs over 48-51 weeks. Other biosimilars had more limited indication-specific studies. This broader, clinical evidence base likely increased prescriber comfort.
Treatment Continuation and Attrition
A critical concern with any medication transition is the potential for treatment disruption. The study found:
- 91% of patients remained on a biosimilar for at least three months post-transition
- Just 4% of patients switched to an alternate, non-adalimumab treatment
This retention rate significantly outperforms published literature on biosimilar transitions. Previous studies using the German national pharmacy claims database showed 20-30% discontinuation rates for adalimumab biosimilars during the same timeframe, depending on disease state.
Research has suggested that up to 84% of biosimilar discontinuations are attributable to the “nocebo” effect — the opposite of the placebo effect, where negative expectations lead to negative outcomes. The substantially lower discontinuation rates in this program suggest that effective patient education and support strategies helped to reduce patient attrition.
Among those who discontinued treatment and switched to alternative therapies, IL-17 inhibitors (primarily Skyrizi® and Taltz®) were the most common alternative treatment class (70%), followed by TNF inhibitors like Enbrel® (15%) and other mechanisms of action, including Orencia® and the JAK inhibitor Rinvoq®.
Figure 1: Humira and Biosimilar Attrition
|
Reason for Discontinuation |
Plan A |
Plan B |
Plan C |
Total |
|---|---|---|---|---|
|
Changed therapy |
9 |
7 |
6 |
22 |
|
Cost or holding therapy |
6 |
9 |
NA |
15 |
|
Patient or provider preference |
1 |
6 |
NA |
7 |
|
Side effects |
1 |
0 |
NA |
1 |
|
No documentation |
0 |
5 |
21 |
26 |
91% remained on a biosimilar three months post-transition.
Figure 2: Biosimilar Transition Summary
Patient Experience and Clinical Impact
Patient-reported outcomes and clinical measures revealed minimal negative impact from the transition:
- 91% of patients reported satisfaction with their biosimilar treatment
- Incidence of adverse events was similar between Humira and biosimilars, with biosimilars showing fewer injection site reactions than Humira
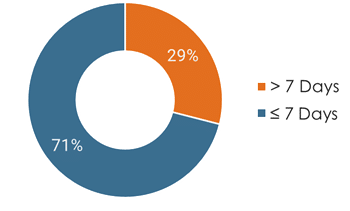
- A majority of patients (67%) experienced no significant gap in therapy during transition, with transitions occurring within seven days
- Of those that experienced a gap in care, a majority (71%) resumed treatment within 14 days
Figure 3: Satisfaction Scores Between Humira and Biosimilars
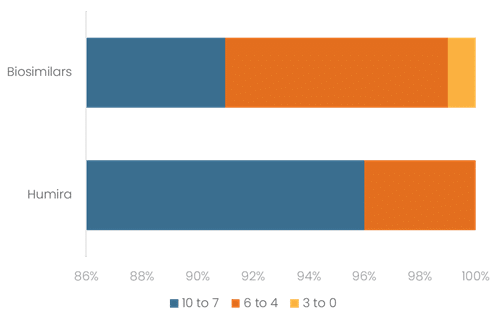
0 reflects low satisfaction; 10 reflects high satisfaction.
Figure 4: Humira to Biosimilar Gap in Therapy Distribution
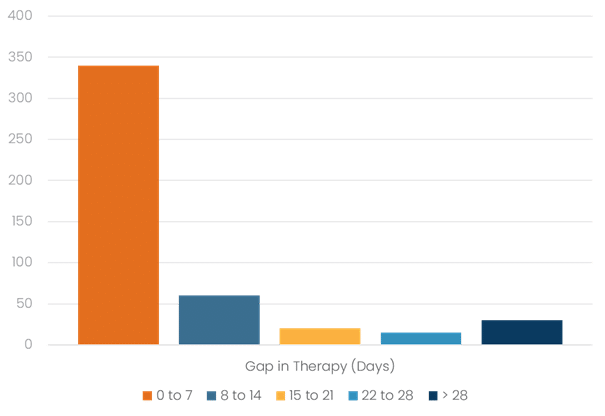
Figure 5: Humira to Biosimilar Gap in Therapy Distribution
Gap analysis showed no statistically significant differences between specialty pharmacies in managing the transition. Average gap days of 7.0 for one client group, 9.7 for another and 7.3 for the third were recorded. Importantly, there was no correlation found between gaps in therapy exceeding 7.0 days and subsequent treatment discontinuation. This suggests that the brief transition period, when applicable, did not negatively impact long-term medication persistence.
These findings suggest that well-managed transitions to biosimilars can occur without compromising patient care or satisfaction. This is a critical factor for providers and health plans considering such programs.
Economic Impact
The economic benefits of this biosimilar transition program were substantial for both patients and health plans:
- Average patient copay decreased nearly 97%, from $4.53 per month with Humira to $0.15 per month with biosimilars (p < 0.001)
- Patients in a coinsurance structure that remained on adalimumab therapy saved an estimated $1,180 per month in out-of-pocket costs
- Participating plans saved $20.79 in per member per month (PMPM) cost before rebates
Figure 6: Out-of-Pocket Costs
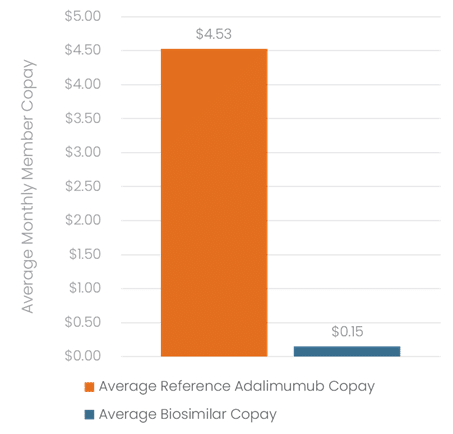
Figure 7: Plan Savings

These patient-level savings are particularly notable when compared to broader market trends. A 2024 JAMA cohort study of 190,364 patients, with 1.7 million claims for seven biologics between 2009 and 2022, found that annual out-of-pocket spending did not decrease after the introduction of biosimilar competition, with costs remaining similar between biosimilars and reference products.
The dramatic 97% reduction in average copays observed in the Navitus adalimumab transition program demonstrates the effectiveness of the specific formulary design and benefit structure implemented. These savings were achieved while maintaining high treatment continuation rates and patient satisfaction, demonstrating that cost reduction need not come at the expense of clinical outcomes.
Key Findings
Several factors contributed to the high success rate of this adalimumab biosimilar transition program:
- Proactive communication: Early notification to patients and providers by the specialty pharmacy about the formulary change, with multiple touchpoints
- Clinical support: Pharmacist availability to proactively address questions and concerns throughout the transition
- Provider education: Clinician outreach to share information about biosimilar efficacy, safety and interchangeability, including education on the “nocebo” effect
- Minimized administrative burden: Coordinated prior authorization processes and prescription transfer to ensure continuity of care
- Financial incentives: Lower out-of-pocket costs for patients choosing biosimilars, complemented by manufacturer support programs
- Coordinated stakeholder approach: Alignment between the pharmacy benefit manager, specialty pharmacy, providers and plan sponsors
The research highlighted the importance of provider engagement to support biosimilar transition. Specifically, physician buy-in is a critical success factor for similar programs. The differences between the three client groups highlighted the impact of the provider as a channel for patient communication, with the client group showing higher retention rates, demonstrating additional provider engagement in the transition process.
The results also highlighted the importance of interchangeability designation for some biosimilars. For products like Simlandi (adalimumab), a branded biosimilar, the FDA’s interchangeability designation allowed pharmacists to make the switch directly, when prescribers did not send a new prescription. This demonstrates the practical value of this regulatory pathway for transition programs.
Broader Implications for Healthcare Systems
The successful implementation of this biosimilar transition program offers several important insights for healthcare stakeholders:
For Payers and PBMs
- Mandatory, formulary-driven biosimilar transitions can effectively increase biosimilar utilization while maintaining clinical outcomes
- Pass-through pricing models that transparently disclose all rebates and fees may better align incentives for biosimilar adoption
- Early communication and education are essential components of successful implementation
For Providers
- Biosimilars demonstrate equivalent clinical outcomes to reference products in
real-world settings - Patient satisfaction remains high after transition when proper support is provided
- The low rate of adverse events supports confidence in biosimilar safety
For Patients
- Stability in out-of-pocket costs between reference product and biosimilar alternatives can be achieved during transitions with access programs
- Treatment efficacy and safety profiles remain consistent with reference products
- Proper support from healthcare teams can ensure smooth transitions
Study Limitations
The researchers acknowledged several limitations to their findings:
- Claim dates may not perfectly reflect when patients administered their medication, potentially affecting gap in therapy calculations
- Limited sample size restricts ability to generalize for some outcome measures
- The relatively short post-transition study period may not reflect long-term outcomes
Despite these limitations, the consistently positive outcomes across multiple measures provide strong real-world evidence supporting biosimilar transitions. These findings highlight the need for continued research and longer-term follow-up studies to fully characterize adalimumab biosimilar utilization and outcomes.
Conclusion
This real-world evidence study demonstrates that well-executed biosimilar transition programs can achieve high rates of successful adoption, while maintaining clinical outcomes, patient satisfaction and continuity of care. The substantial cost savings for both patients and health plans suggest that broader implementation of such programs could significantly impact healthcare expenditures without compromising quality of care.
The findings support a formulary-driven approach to biosimilar adoption when accompanied by thorough education, communication and support. As more biosimilars enter the market, including for other high-cost biologics, the lessons from this adalimumab transition program provide a valuable roadmap for healthcare stakeholders seeking to balance cost management with optimal patient care.
References
Rubin DT, Mittal M, Davis M, Johnson S, Chao J, Skup M. Impact of a patient support program on patient adherence to adalimumab and direct medical costs in Crohn’s disease, ulcerative colitis, rheumatoid arthritis, psoriasis, psoriatic arthritis, and Ankylosing Spondylitis. J Manag Care Spec Pharm. 2017;23(8):859-867. doi:10.18553/jmcp.2017.16272
Jin R, Kruppert S, Scholz F, et al. Treatment persistence and switching patterns of adalimumab biosimilar ABP 501 in European patients with rheumatologic diseases. Rheumatol Ther. 2024;11(3):523-537. doi:10.1007/s40744-024-00647-4
Jeremias S. Nocebo effect is difficult to diagnose in patients switched to biosimilars. The Center for Biosimilars. March 17, 2020. Accessed April 7, 2024. https://www.centerforbiosimilars.com/view/nocebo-effect-is-difficult-to-diagnose-in-patients-switched-to-biosimilars
Feng K, Russo M, Maini L, Kesselheim AS, Rome BN. Patient out-of-pocket costs for biologic drugs after biosimilar competition. JAMA Health Forum. 2024;5(3):e235429. doi:10.1001/jamahealthforum.2023.5429
Stay Informed and Connected
Receive expert insights, healthcare tips, and important updates on pharmacy benefits, drug recalls, and more—straight to your inbox.
Navigating with a trusted partner
Now Available: 9th Annual Drug Trend Report
Our Drug Trend Report provides a clear view of the trends shaping pharmacy benefits today, along with strategies that are delivering real savings without compromising care.