Everything You Need to Know About the Drug Development Process
New medications entering the market can impact both your organization’s pharmacy spend and your members’ cost share.
With thousands of drugs in development, it’s important to stay ahead of the curve and to pay attention to the select few that are likely to get FDA approval, hit the market and make a splash.
Luckily, our team of pharmacists and other experts has you covered. In this blog series, we’ll explore some of the most important drugs launching this year or nearing the end of their development cycle and present how these may impact you and your members. But before we do that, let’s take a quick look at what it takes to develop a new drug.
DRUG DEVELOPMENT: HOW IT WORKS
In order to know when a new medication is coming to market, it is important to understand the process for developing a new drug. Bringing a new drug to market is a lengthy and complex process that takes an average of 10 years, including 6-7 years of clinical trials.1 The average cost for developing a new drug is $2.6 billion and only about 12% of drugs that enter clinical trials will ultimately be approved for sale.1 In the United States, the Food and Drug Administration provides regulatory oversight and undertakes the final review of each new drug.
The Drug Development Process
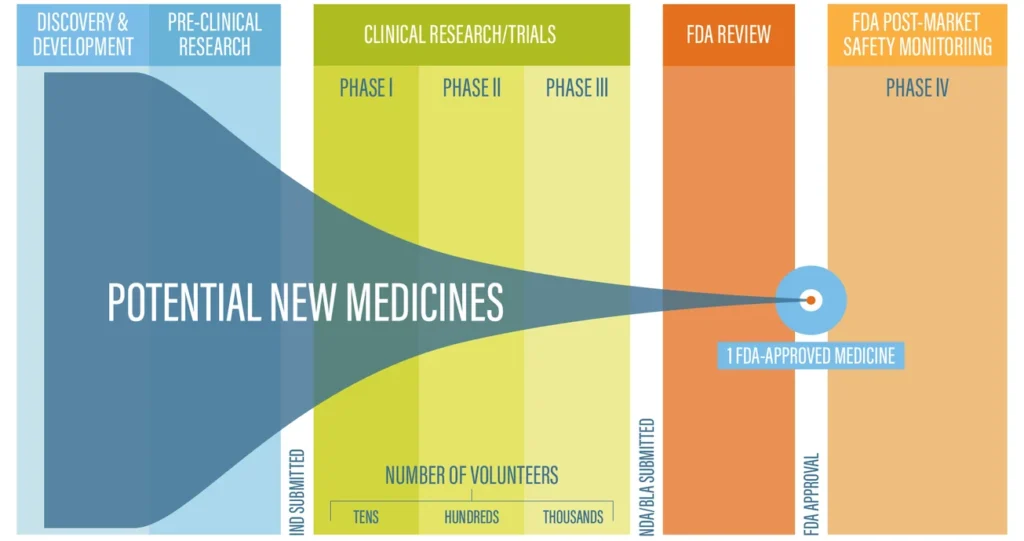
Here is a quick summary of what happens at each stage:
| Discovery & Development | Narrows down thousands of potential drugs |
| Pre-Clinical Research | Determines if the drug is safe to test on people |
| Clinical Research/Trials | |
| Phase I | Tests safety Fewer than 100 volunteers, usually healthy, participate Length: Several months ~70% of drugs move to Phase II |
| Phase II | Assesses safety and efficacy Several hundred volunteers with a condition participate Length: Months to 2 years ~33% of drugs move to Phase III |
| Phase III | Demonstrates safety and efficacy 300-3000 volunteers with a condition participate Length: 1 to 4 years 25-30% drugs move to FDA Review |
| FDA Review | FDA reviews the application and decides whether to approve the drug for sale; this process usually takes 10 months, although drugs flagged for priority review may be approved in 6 months |
| FDA Post-Market Safety Monitoring | Ongoing reporting and data collection after the drug comes to market |
For this series, we will focus on recently approved drugs that are undergoing FDA review or that are approaching the end of Phase III clinical trials. In some instances, we will also highlight promising drugs that are in Phase II trials or just beginning Phase III trials. While these won’t be coming to market in 2020, they have the potential to make a real impact in coming years.
Join us next week for a detailed overview of the most important specialty medications of 2020.
Biopharmaceutical Research & Development: The Process Behind New Medicines. Pharmaceutical Research and Manufacturers of America. http://phrma-docs.phrma.org/sites/default/files/pdf/rd_brochure_022307.pdf. Published May 2015. Accessed January 8, 2020.
Stay Informed and Connected
Receive expert insights, healthcare tips, and important updates on pharmacy benefits, drug recalls, and more—straight to your inbox.
Navigating with a trusted partner
Now Available: 9th Annual Drug Trend Report
Our Drug Trend Report provides a clear view of the trends shaping pharmacy benefits today, along with strategies that are delivering real savings without compromising care.








